General information
According to St Vincent’s Hospital, Melbourne, (2018) and Di Bella et al. (2018), the Biopen is handheld 3D printing device that has been developed collaboratively by researchers in Australia. The invention can hold new stem cells which can then be applied directly to damaged bone or cartilage of a patient by surgeons.
The Biopen’s purpose is to prevent the onset of osteoarthritis by speeding up the stem-cell repair of patients because the current treatment does not provide the desired long-term effects.
Scope TV (Biopen 2017)
Technical and historical information
The Biopen is based on bioprinting which is defined by Di Bella et al. (2018, p. 611 ) as ‘the simultaneous deposition of structural biomaterials and living cells’. The Biopen itself is a hand-held 3D bioprinting device for surgeons to apply living cells and biomaterials (bioscaffold) directly onto the damaged bone or cartilage to accurately to correct the injury (Di Bella et al. 2018). According to BioMedTech Horizons (2018), this is done by ‘isolating the stem cells from the patient, loading these into a gel scaffold then printing new cartilage using the Biopen directly onto the injury’ then hardening the repair by using UV light.
Hardware
Di Bella et al. (2018) states that the Biopen is made of medical grade ABS-like material and titanium 6AI4V alloy.
The system is made up of four components:
- Scaffold chambers that contain bio-ink (Moroni, 2018, p. 3)
- A multi-inlet extruder nozzle
- A light source to catalyse phase transformation of the ink
- A motorised extrusion system
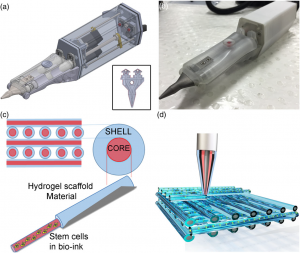
Figure 1 (a) Design of the Biopen, (b) Photograph of the Biopen, (c) Representation of the core/shell distribution, (d) Representation of the multiple layer three-dimensional printed block in a criss-cross pattern (Di Bella et al. 2018, fig 1, p. 162).
Software
All of the components of the Biopen were designed using Solidworks CAD software. 3D print files are then used to create the medical structure required according to O’Connell et al. (2016).
The history of the Biopen
In 1985, Dr Fung at the University of California in San Diego introduced the term tissue engineering then in 1993, the approach of tissue fabrication was defined by Langer and Vancati (cited in Gao and Ciu 2016, p. 203).
The current process of treating tissue damage is by creating an exact match of the tissue to be replaced then applying to the bone or cartilage during surgery. It is technically unachievable to develop a right match by using computed tomography, and magnetic resonance imaging scans say Di Bella et al. (2018). Also, using the current treatment makes it impossible to fully repair tissue damage as explained by Lewis et al. 2006 and Tetteh et al. 2012 (cited in Di Bella et al, 2018, p. 611). These authors suggest that the treatment currently available to treat damaged bones and cartilage have a limited ‘effectiveness’.
Di Bella et al. (2018) writes the first Biopen research article was released in August 2016 demonstrating this new technology to treat bone and cartilage damage. The University of Melbourne had undertaken research, St Vincent’s Hospital Melbourne, University of Wollongong and Swinburne University of Technology about the developing the Biopen technology. Successful trials had been conducted on sheep with the next step being human trials.
According to MTP Connect (2018), in April 2018, the Biopen was one of eleven projects to receive $35 million in funding from the Australia government to move forward and begin producing all that is required to move the Biopen into use within the medical industry.
Figure 2 Biopen receives funding to boost health innovations in Australia (St Vincent’s Hospital, Melbourne, (2018)
Impact of Technology
Bella et al. (2018) advise the Biopen aims to have a significant impact on both larger society and healthcare. It will be a disruptive technology that addresses both financial and health issues suffered by patients with bone or cartilage damage. The technology will be disruptive by providing treatment that has long-term results as opposed to the current treatment available. It will also address the financial burden on both the patient and healthcare system because patients suffering injuries to bone or cartilage will receive a treatment that is quick, effective and with minimal need to have repeat treatment. The need for such a technology is supported by Di Bella et al. (2018, p. 611) who states that ‘joint injuries cause substantial pain and loss of function, and may result in osteoarthritis at considerable cost to the patient and healthcare system’.
Bibliography
BioMedTech Horizons 2018, Projects, retrieved 25 April 2018,
<https://www.mtpconnect.org.au/biomedtechhorizons>
Di Bella, C, Duchi, S, O’Connell, CD, Blanchard, R, Augustine, C, Yue, Z, Thompson, F, Richards, C, Beirne, S, Onofrillo, C, Bauquier, SH, Ryan, SD, Pivonka, P, Wallace, GG, Choong, PF 2018, ‘In situ handheld three-dimensional bioprinting for cartilage regeneration’, Tissue engineering and regenerative medicine, 12(3), p. 611, retrieved 23 April 2018, Academic OneFile database.
Gao, G, Cui, X 2016. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnology Letters, vol. 38, no. 2, p. 203, 9 p, retrieved 21 April 2018, Academic OneFile database.
Moroni, L, Boland, T, Burdick, JA, De Maria, C, Derby, B, Forgacs, G, Groll, J, Li, Q, Malda, J, Mironov, VA, Mota, C, Nakamura, M, Shu, W, Takeuchi, S, Woodfield, TBF, Xu, T, Yoo, JJ, Vozzi, G 2018, Biofabrication: A guide to technology and terminology. Trends in Biotechnology, vol 36, no. 4, pp. 384-402, retrieved 23 April 2018, ScienceDirect database
MTP Connect 2018, $10m in funding awarded to 11 projects to boost health innovation, retrieved 24 April 2018, < https://www.mtpconnect.org.au/Story?Action=View&Story_id=105>
O’Connell, C, Di Bella, C, Thompson, F, Augustine, C, Beirne, S, Cornock, R, Richards, CJ, Chung, J, Gambhir, S,Yue, Z, Bourke, J, Zhang, B, Taylor, A, Quigley, A, Kapsa, R, Choong, P, Wallace, GG 2016. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication, vol 8, no. 1, retrieved 23 April 2018, Google Scholar
St Vincent’s Hospital, Melbourne 2018, BioPen receives funding to boost health innovations in Australia, retrieved 23 April 2018, <https://www.svhm.org.au/newsroom/announcements/biopen-receives-funding-to-boost-health-innovations-in-australia>
Video
Biopen, YouTube, Scope TV, 4 December 2017, retrieved 23 April 2018, < https://www.youtube.com/watch?v=tVCCioe-yRU>
Cartilage Regeneration: An ACES/ANFF case study, YouTube, ACESElectromaterials, 24 January 2017, retrieved 23 April, 2018, <https://www.youtube.com/watch?v=cG06AzKRS2c&feature=youtu.be>
Images
In situ handheld three‐dimensional bioprinting for cartilage regeneration 2016, (a) Design of the Biopen, (b) Photograph of the Biopen, (c) Representation of the core/shell distribution, (d) Representation of the multiple layer three-dimensional printed block in a criss-cross pattern, image, retrieved 25 April 2018, Academic OneFile database
St Vincent’s Hospital Melbourne, BioPen receives funding to boost health innovations in Australia 18 April 2018, photograph, retrieved 25 April 2018, <https://www.svhm.org.au/newsroom/announcements/biopen-receives-funding-to-boost-health-innovations-in-australia>